When I was first learning about science, everyone was confident about the existence of atoms but no one ever expected to "see" them. That all changed in the 1980s with the invention of scanning-probe microscopies at IBM Zurich, first scanning tunneling microscopy, then atomic force microscopy and others.
But even in those earlier days, textbooks showed a few pictures of atoms in real space. Those pictures came from a field emission microscope, in which the strong electric field at an ultra-sharp metallic tip rips electrons out of the atoms. Because the field lines diverge rapidly from the tip, the pattern of electrons from different atoms spreads out rapidly until an enlarged version of the atomic arrangement can be directly visualized on a phosphor screen.
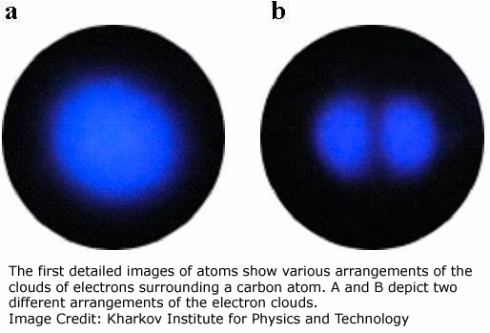
Now Ukrainian researchers have adapted this venerable technique in an upcoming paper in Physical Review B to look at the different arrangements of electrons within a single atom. Yes, that looks like s and p orbitals. Yet another thing I never thought I'd see! What a world, what a world.




No comments:
Post a Comment